Therapeutic apheresis: overview
AKA: therapeutic plasma exchange (TPE, PLEX, PEX)
A general overview of the principles of therapeutic apheresis in pediatric patients
Overview
-
Blood is made up of red blood cells (~45%), plasma (~55%), and the buffy coat* (<1%), which contains most of the white blood cells and platelets
- Plasma is about 91.5% water, 7% proteins, and 1.5% other solutes
FYI
It is called the buffy coat because it is buff colored 🟨
- Plasma is about 91.5% water, 7% proteins, and 1.5% other solutes
-
Apheresis is the separation and removal of blood components (e.g., cells, antibodies, immune complexes, or other target substances) that are too large to be removed with dialysis
- Can be used as a therapy or for harvesting a component of the blood
- Blood from the patient perfuses the extracorporeal circuit, where the blood is separated into components and a selected component (e.g., the plasma) is removed
- Blood separation can be accomplished by centrifugation or by filtration using a selective membrane
- If a large volume is removed, as with plasma exchange, that volume is replaced during the procedure with albumin, fresh frozen plasma (FFP), or some combination of the two
- For smaller volumes, normal (0.9%) saline may be given to replace the volumes removed
Methods: membrane vs centrifugation
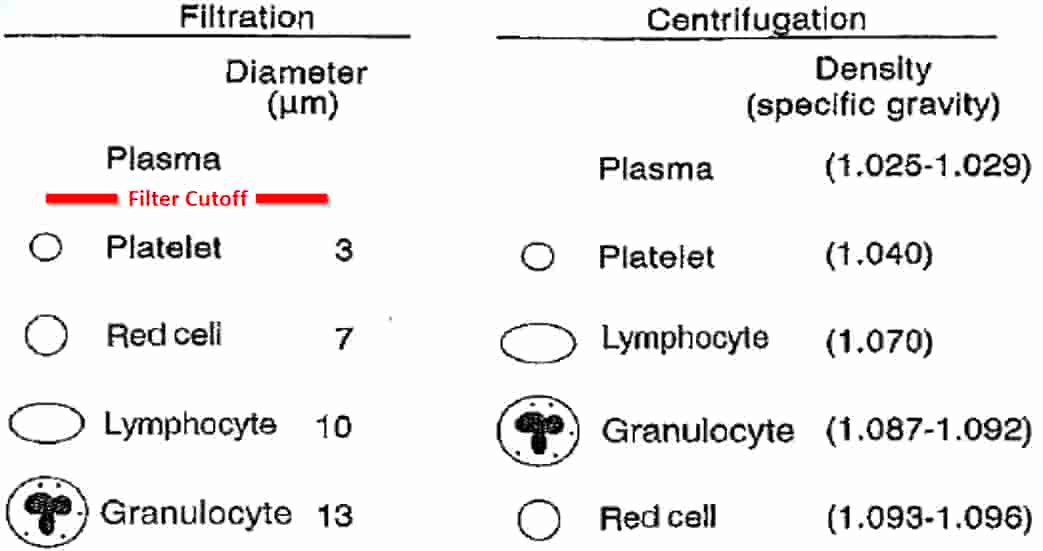
Membrane filtration
- Blood component separation by molecular size
- Hollow fiber capillary membrane employs much larger pores than the filters used in dialysis (up to 0.2 µm), which allows proteins to pass through (e.g., albumin, immunoglobulins, immune complexes, complement factors, lipoproteins, endotoxin)
- The pores, which are about 30 times bigger than the pores in conventional high-flux
filters, are not big enough to allow cellular elements to pass through the membrane - Membrane filtration cannot be used for cytapheresis
- The pores, which are about 30 times bigger than the pores in conventional high-flux
- Membranes used in TPE have very high sieving coefficients (i.e., high percentage of substance removed as it passes through the filter) for plasma components:
- Albumin 0.97, IgG 1.00, Apolipoprotein B 0.95, IgM 0.92
- The membranes are nonselective and will remove any component of the plasma that will fit through its large pores
- To avoid filter clogging, the filtration fraction (% of plasma extracted each time it passes through the filter) is limited to 30-35% of the plasma
- High blood flows (100-250 mL/min, typically ~150 mL/min) are required to limit the filtration fraction
- The entire blood volume typically needs to be processed 3-4 times to remove enough plasma
Centrifugation
- Blood component separation by density (specific gravity)
- Most commonly, blood from the patient is anticoagulated with citrate and is delivered continuously into a spinning centrifuge which separates blood components into layers in the following order:
- Plasma → buffy coat (platelets → lymphocytes → monocytes → granulocytes) → red blood cells
- Machine can then selectively remove a particular layer
- Can use this technique to perform plasmapheresis or cytapheresis
- Separation is not perfect; some cells and platelets will be removed in the plasma
- The remaining blood as well as the plasma replacement fluid are continuously infused back into the patient
| Centrifugation | Membrane filtration |
|---|---|
| Not limited to plasma removal | Unable to remove cellular components |
| Can sometimes be performed using peripheral IV access, even in children | Central venous access is necessary to maintain blood flows |
| Higher extracorporeal blood volumes (~180 mL) | Lower extracorporeal blood volumes (~125 mL) |
Therapeutic plasma exchange (TPE, PLEX)
- Most commonly performed apheresis procedure
- The goal is to rapidly remove pathogenic substances from the plasma, which may include autoantibodies, cytokines, or abnormal proteins (e.g., complement components)
note
All substances in the plasma are removed, including clotting factors as well as medications like antibiotics and vasopressors
- Over a period of 1.5-2 hours, the majority of a patient’s plasma is removed and replaced with a physiologic fluid (albumin, fresh frozen plasma [FFP], or a mixture of the two) in order to maintain intravascular volume status
- TPE is not dialysis
- Unlike dialysis, TPE clears large particles and can remove protein-bound particles
- TPE does not treat the underlying disease
American Society for Apheresis (ASFA) guidelines
The American Society for Apheresis (ASFA) published evidence based guidelines for the use of therapeutic apheresis in a wide variety of disorder
- The guidelines provide a 1-2 page summary regarding the disorder and the rationale for therapeutic apheresis, including:
- A brief description of the disease and its incidence
- Overview of management
- Disease category and evidence rating
- Includes the number of trials/case reports and the total number of patients included in each type
- Rationale for using apheresis in a given condition
- Technical notes on the apheresis procedure:
- Which modality to use, how often to do the apheresis procedure, how much plasma volume to exchange in each procedure, and what replacement fluid is recommended
- How to assess treatment response and when to discontinue therapy
ASFA Categories
Disorders are categorized based on how well the evidence supports the use of apheresis:
- Category 1: apheresis is accepted as first-line therapy, either as primary standalone treatment or in conjunction with other modes of treatment
- Category 2: apheresis is accepted as second-line therapy, either as standalone treatment or in conjunction with other modes of treatment
- Category 3: optimum role of apheresis therapy is not established; decision making should be individualized
- Category 4: published evidence demonstrates or suggests apheresis to be ineffective or harmful; if apheresis is performed for a category 4 condition, it should be done as part of an IRB-approved study
Category 1 recommendations
Based on the ASFA guidelines, apheresis is appropriate for use as a first-line therapy in the following conditions, though the modality of apheresis will vary by condition:
- Acute inflammatory polyneuropathy (Guillain-Barré syndrome, GBS) [rarediseases.org]
- Includes acute inflammatory demyelinating polyradiculoneuropathy (AIDP, the most common form of GBS)
- Acute liver failure (ALF)
- ANCA-associated vasculitis (AAV) in patients with severe AKI due to rapidly progressive glomerulonephritis (RPGN) or in patients with diffuse alveolar hemorrhage (DAH)
- Includes microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA, FKA Wegener syndrome), and renal-limited vasculitis (RLV)
- Anti-glomerular basement membrane (anti-GBM) disease (Goodpasture syndrome) in non-dialysis dependent patients or patients with diffuse alveolar hemorrhage (DAH)
- Catastrophic antiphospholipid syndrome (CAPS)
- Chronic inflammatory demyelinating polyradiculopathy (CIDP; AKA chronic inflammatory demyelinating polyradiculoneuropathy) [rarediseases.org]
- Cutaneous T-cell lymphoma (CTCL) with mycosis fungoides (Sézary syndrome), erythrodermic [rarediseases.org | physician guide]
- Familial hypercholesterolemia (FH), homozygous (HoFH) [rarediseases.org | physician guide]
- Focal segmental glomerulosclerosis (FSGS), recurrence after transplant
- Hereditary hemochromatosis [rarediseases.org]
- Hyperviscosity in hypergammaglobulinemia
- Myasthenia gravis, acute/short-term treatment [rarediseases.org]
- N-methyl-D-aspartate (NMDA) receptor antibody (anti-NMDAR) encephalitis [rarediseases.org]
- Paraproteinemic demyelinating neuropathies, IgG/IgA/IgM
- Polycythemia vera [rarediseases.org]
- Sickle cell disease, acute stroke/stroke prophylaxis [rarediseases.org]
- Thrombotic microangiopathy (TMA), if caused by factor H autoantibody, ticlopidine, or thrombotic thrombocytopenic purpura (TTP [rarediseases.org])
- Transplantation
- Liver, desensitization, living donor (ABO incompatible)
- Kidney, antibody mediated rejection (ABMR)
- Kidney, desensitization, living donor (ABO compatible or ABO incompatible)
- Wilson disease, fulminant [rarediseases.org]
Category 4 recommendations
ASFA recommends against the use of apheresis in the following conditions, except in the setting of a trial:
- Amyotrophic lateral sclerosis (ALS) [rarediseases.org]
- Dermatomyositis/polymyositis [rarediseases.org]
- Inclusion body myositis [rarediseases.org]
- POEMS syndrome (Takatsuki syndrome) [rarediseases.org]
- Rheumatoid arthritis
- Schizophrenia
Apheresis Modalities
Types of apheresis procedures include:
- Plasmapheresis (therapeutic plasma exchange [TPE, PLEX])
- Leukapheresis
- Photopheresis
- Red cell pheresis (erythrocytapheresis, red blood cell exchange)
- Plateletpheresis (thrombocytapheresis, platelet depletion)
- Stem cell collection/harvesting
- Collection of hematopoietic progenitor cells (HPCs), also known as peripheral blood stem cells (PBSCs)
- Immunoadsorption/selective adsorption
- Low density lipoprotein (LDL) apheresis
Plasma exchange kinetics
- Plasma exchange can only remove substances that are in the intravascular compartment
- The approximate intravascular distribution of common proteins: IgG (excluding IgG3 subclass) 45%, IgA 42%, IgM 76%, albumin 40%, fibrinogen 80%
- For example, exchanging 1.5 plasma volumes of IgG will decrease total body IgG by ~35%
- The approximate intravascular distribution of common proteins: IgG (excluding IgG3 subclass) 45%, IgA 42%, IgM 76%, albumin 40%, fibrinogen 80%
- Multiple treatments are typically required because substance levels recover
- Antibody synthesis may be increased after plasma exchange
- Underscores the need for immunosuppression when treating autoimmune diseases with PLEX
- Acute removal of substances from plasma may disrupt the balance of synthesis and catabolism
- E.g., as IgG (excluding IgG3) levels decrease, IgG catabolism (both total and fractional) also decreases and the half-life (t1/2) increases
- Some substances rapidly redistribute into the intravascular space (even during the procedure)
- Other substances re-equilibrate from other compartments into the intravascular space
- E.g., re-equilibration for IgG occurs over 1-2 days, so TPE is typically performed every other day for IgG-mediated diseases
- Antibody synthesis may be increased after plasma exchange
Diminishing returns
- Removal of plasma components follows a negative logarithmic scale
- A fixed proportion of the substance is removed (65-70%), and its concentration drops over time as it is being diluted by the replacement fluid
- Exceeding 1.0-1.5x plasma volume exchanged in a single treatment is rarely necessary
- Extends treatment time and increases risks of complications while providing little benefit
- Under idealized conditions, exchange of the first plasma volume (a 1x plasma volume exchange) removes 63% of a solute, a second plasma volume removes 23%, and a third plasma volume removes only 9% [PMID 24178973]
- 1.2 x plasma volume exchange removes ~70%
- 1.5x plasma volume exchange removes ~77.7%
note
These are estimations, and not true for all substances
- E.g., small organic and inorganic molecules (uric acid, calcium, potassium) change very little (if at all) due to rapid re-equilibration into the intravascular compartment and/or active homeostasis
Prescribing: Basics
The role of a pediatric nephrologist as the apheresis prescriber is to:
- Help to weigh the risks and benefits of therapeutic apheresis for each patient
- This includes determining the appropriateness of therapeutic apheresis for the patient’s condition
- Ensure a plan is in place to manage the underlying process
- Develop a treatment plan before starting therapy
- Manage the therapeutic apheresis procedure to prevent and mitigate adverse effects
- Monitor the effects of the therapy
Developing a treatment plan
- Does the patient have appropriate access?
- What is the goal of the therapy?
- What plasma volume will be used?
- The plasma volume is calculated based on the patient’s size and hematocrit
- A prescribed multiple of the plasma volume is filtered during the treatment
- How will the circuit be primed?
- How often will treatments be performed?
- Typically daily or every other day, as re-equilibration of substances takes 2 days
- How many treatments will be performed?
- Determining success/failure: how will the effects of treatment be measured? (Biomarkers, physical exam findings?)
- Defining an endpoint: when will treatment be stopped?
- What medications may be affected?
- Communication with the primary team and pharmacy is essential
- What is the anticoagulation plan?
- What strategies will be used to mitigate the metabolic risks?
Priming solution
- Options include: blood, albumin, or normal saline (NS)
- Blood prime: used if extracorporeal blood volume (ECV) >10% of estimated blood volume (EBV)
- As a general rule, usually use blood prime if <10 kg
- For blood prime, we mix 140 mL of Adsol-preserved PRBCs (Hct ~55%) with 100 mL of albumin to bring Hct close to physiologic (~32%)
- NS prime: used if ECV ≤10% of EBV and hemodynamically stable
- Albumin prime: used if ECV ≤10% of EBV but hemodynamically unstable
- Albumin does contain aluminum, so if being used long-term can result in aluminum accumulation (evaluated every 6 months)
Plasma exchange prescription
Calculate the plasma volume (PV)
- Ensure hematocrit (Hct) has been checked on the day of treatment
- Estimated blood volume (EBV): kidney.wiki blood & plasma volume calculator
- For infants, EBV ≈ 80 mL/kg
- For older children, EBV ≈ 70 mL/kg
- Plasma volume (PV) = EBV * (1 - Hct)
- Number of exchanges in a given session, as a multiple of the patient’s plasma volume:
- E.g., 1.0x PV, 1.5x PV, 2.0x PV
- Unless specifically indicated for the patient’s condition, 1.0x PV is recommended
- Total exchange volume (for a given session) = plasma volume * number of exchanges
Replacement fluid
- 5% Albumin
- Consider adding calcium to prevent hypocalcemia
-
Some centers also add potassium to prevent
- Fresh frozen plasma (FFP)
- If part of therapy is replacing factors that are absent (e.g., in TTP)
- Also, if monitoring reveals fibrinogen levels are low
- If part of therapy is replacing factors that are absent (e.g., in TTP)
Anticoagulation
- If using FFP: ACDA at 1.0 x BFR, citrate at 1.0 x ACDA rate
- If using albumin: ACDA at 1.5 x BFR, citrate at 0.4x ACDA rate

